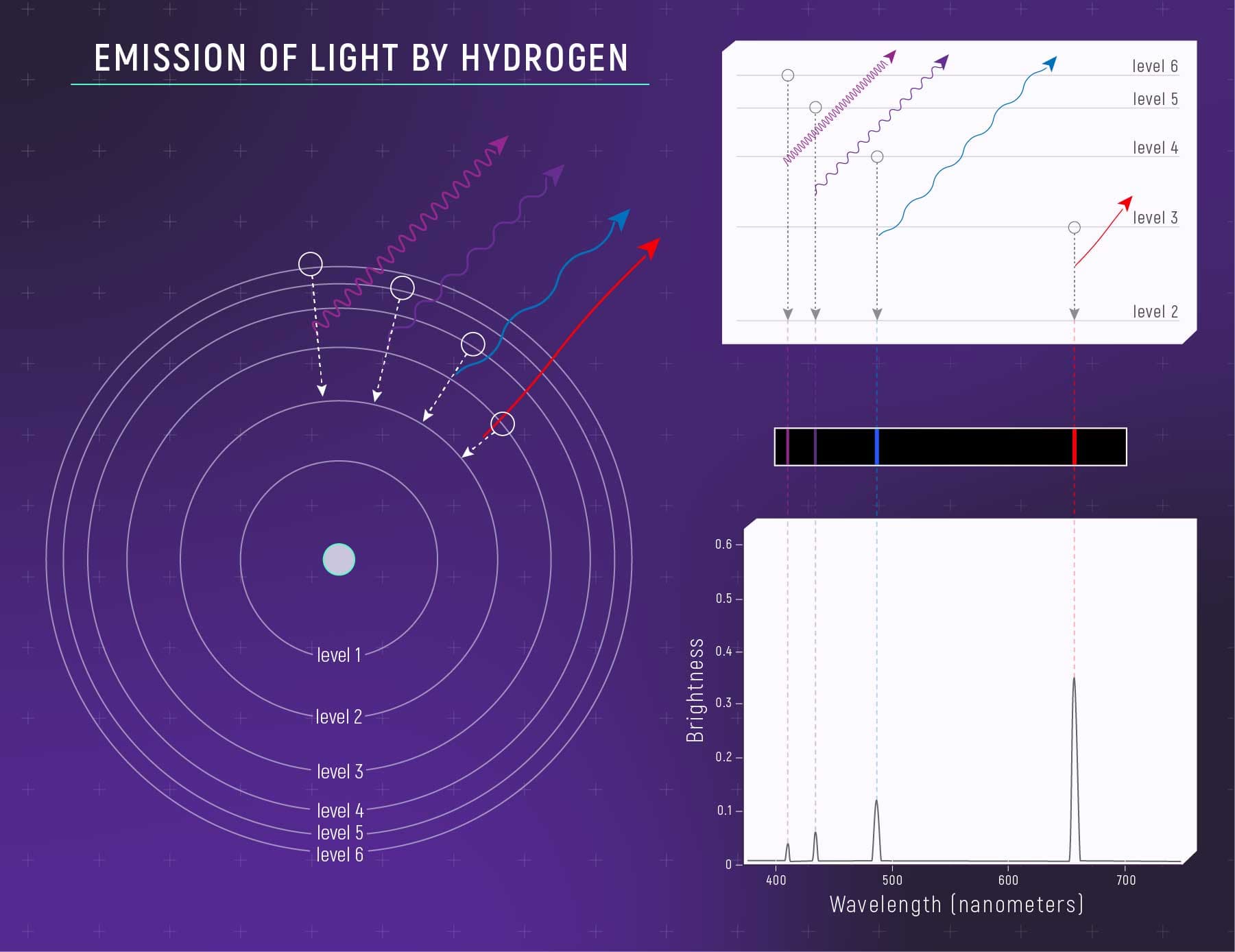
Gases emit unique colors when heated due to different electron arrangements
When electrons within an atom drop to a lower energy level closer to the nucleus, they lose energy and produce light with energy equal to the difference between the two energy levels. Electrons can also move up to higher energy levels if they absorb light with appropriate energy.