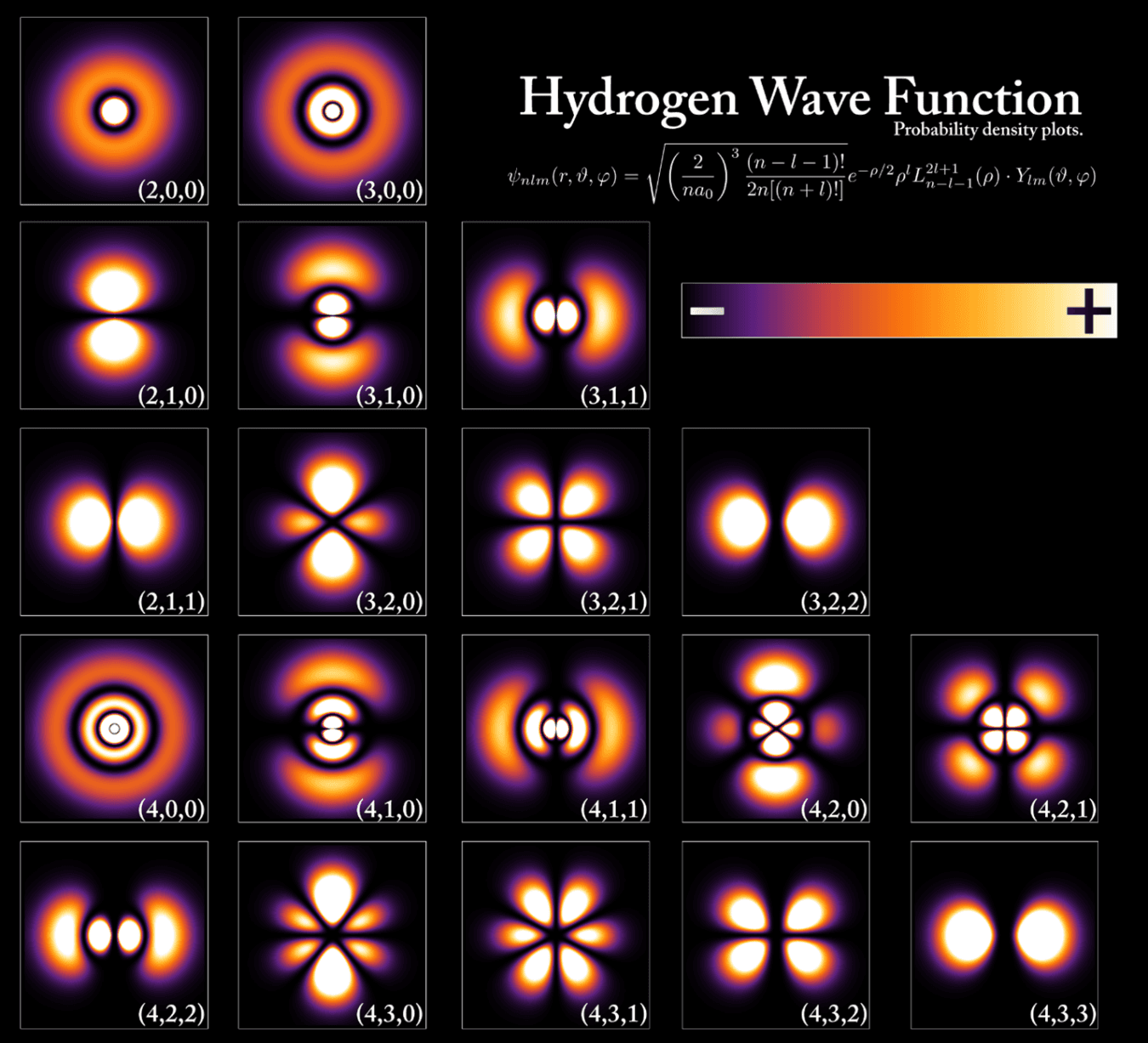
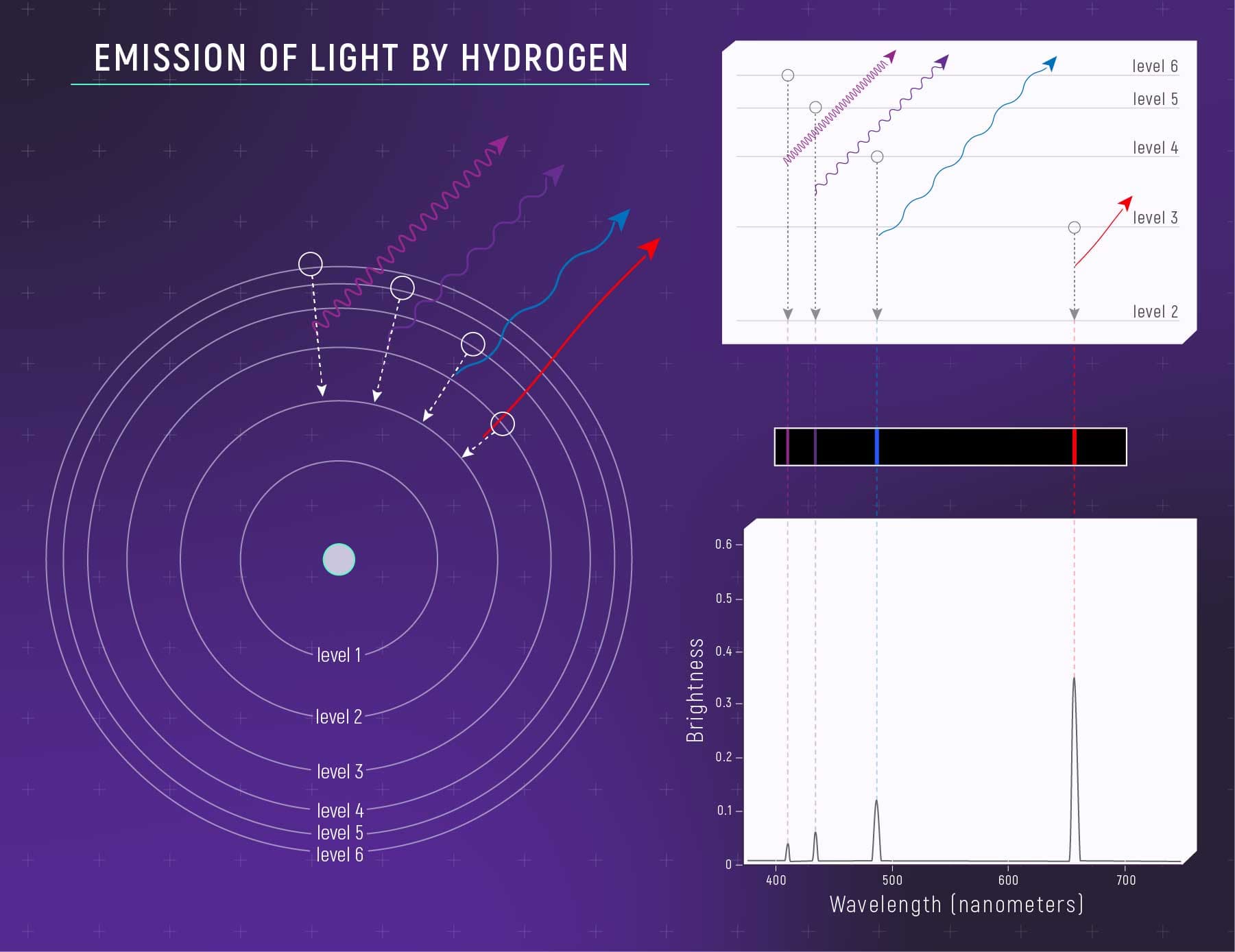
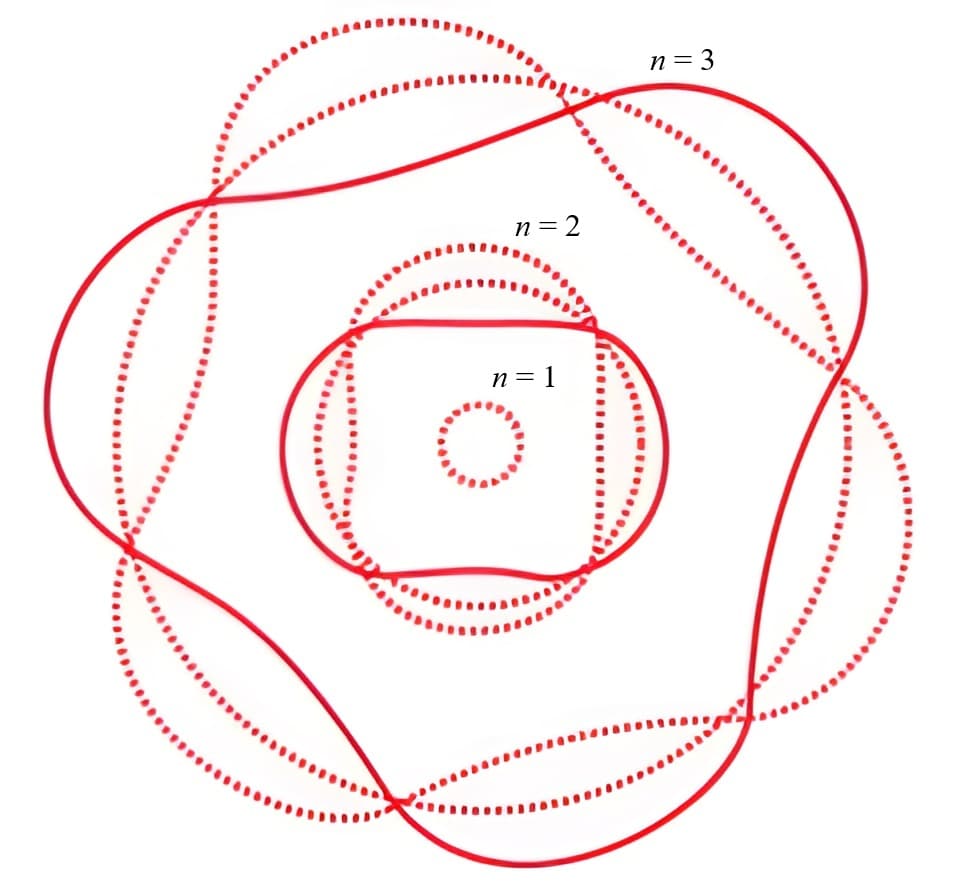
Quantum mechanics explains how particles behave like both matter and waves
In the quantum world, particles such as electrons are “quantized,” meaning they can only hold specific values of energy or momentum. These particles also behave like waves, with their wave function predicting where they might be found at any moment as probability clouds called orbitals.