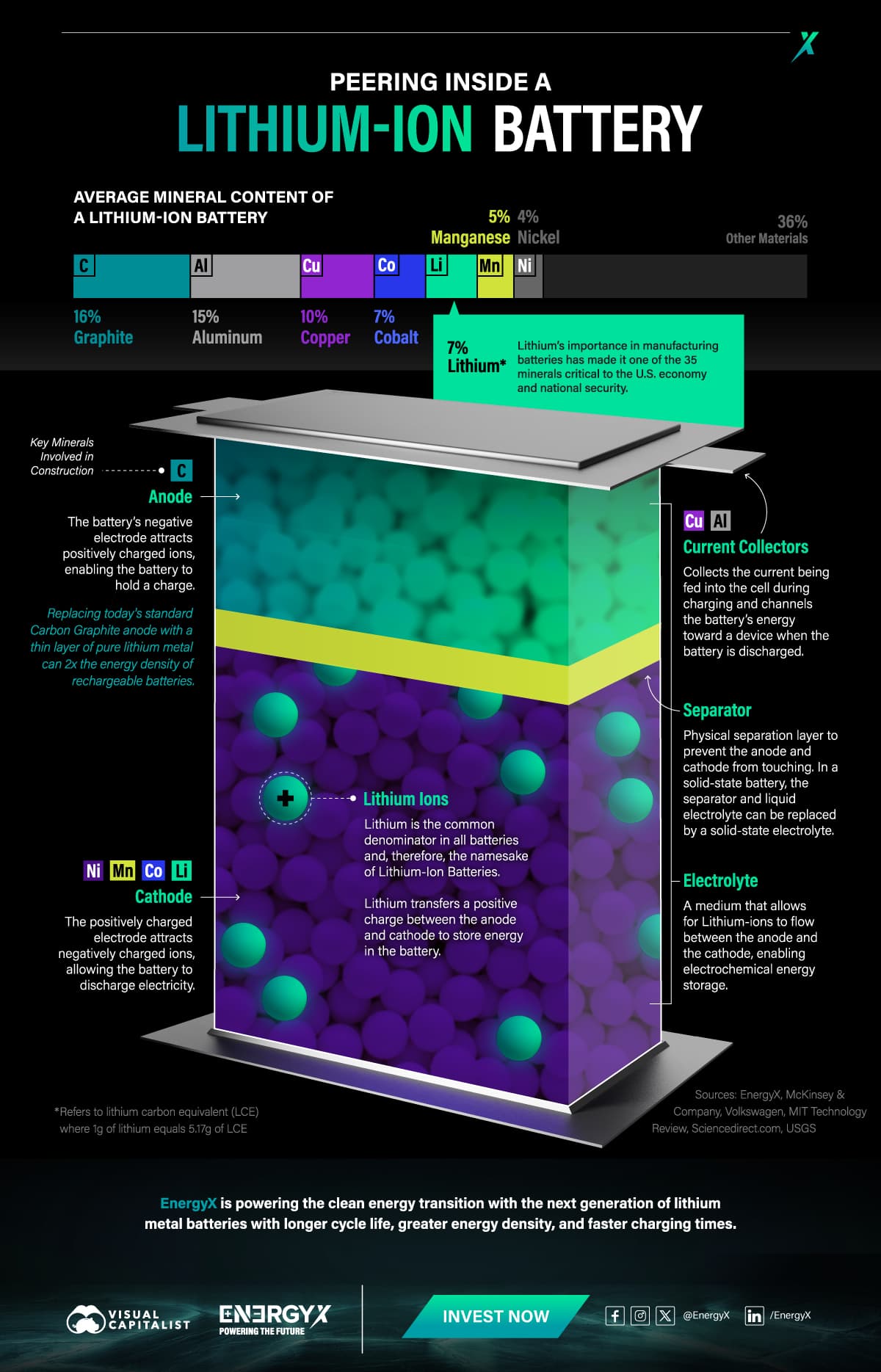
Batteries store energy in chemical bonds that can be turned into electricity
To achieve a more stable, nonreactive state, many elements in nature are reactive and will seek to lose electrons via oxidation or gain electrons through reduction reactions. By pairing an oxidizing chemical with a reducing chemical in a battery, electrons will naturally flow between them, producing an electric current.